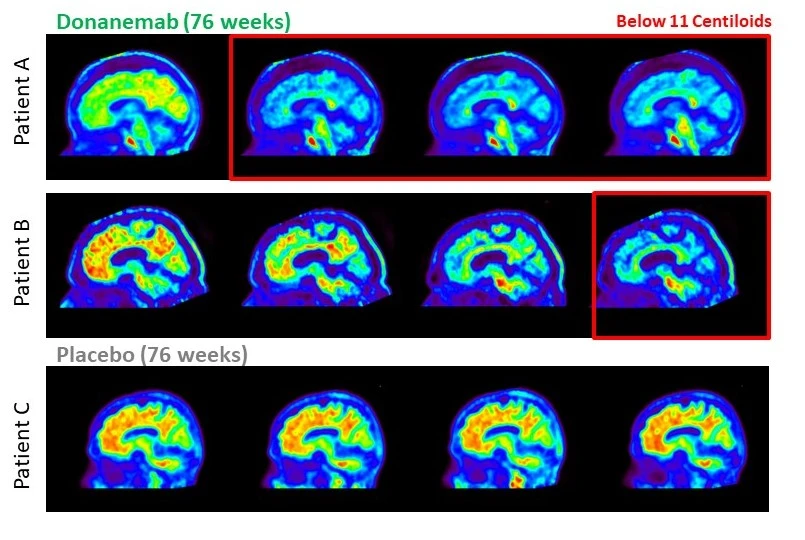
On July 17th, researchers from the TRAILBLAZER-ALZ2 randomized control trial of Donanemab shared the results of their study in parallel to the full publication of their findings. These results represent another remarkable development in Alzheimer’s research. This potential new treatment is showing significant capability in slowing the progression of Alzheimer’s disease, particularly in its early stages.
Donanemab: A Significant Development in Alzheimer’s Research
Alzheimer’s disease, due to its complex nature, has long been a challenging condition to treat. However, recent studies on Donanemab, an experimental drug, have presented promising results that could reshape our approach to treating this condition, especially in its early stages.
The Clinical Impact of Donanemab: Understanding the Data
A recent study involving 1,736 participants demonstrated that Donanemab has a significant impact on slowing the progression of Alzheimer’s disease. In this study, 47% of participants with early-stage Alzheimer’s who received Donanemab showed no progression of the disease on certain measures after a year, compared to 29% who were given a placebo. It is important to note, however, that the effectiveness of Donanemab varied among participants, particularly those in later stages of Alzheimer’s or those carrying a genetic variation known as APOE4, which increases the risk of Alzheimer’s.
The Mechanism of Donanemab: Targeting Amyloid
Donanemab functions by targeting a protein known as amyloid, which accumulates in the brains of persons with dementia due to Alzheimer’s disease and is believed to contribute to neuronal damage in Alzheimer’s. Moreover, the study revealed that Donanemab was particularly effective in patients exhibiting low levels of another protein named tau, which tends to increase as Alzheimer’s progresses and is most closely tied to cognitive impairment.
Recurring Theme: The Importance of Early Intervention
While the research on Donanemab is promising, the study highlights the critical need for early diagnosis and intervention in Alzheimer’s. Early-stage patients demonstrated the most significant benefit from Donanemab, underscoring the necessity for prompt recognition and response to symptoms of cognitive impairment as well as developing innovative health system solutions that can support timely diagnosis and initiation of treatment.
An Inevitable Question: Which Amyloid-Targeting Drug Is Better?
The published results of the Donanemab trial are especially notable given the recent full FDA approval of Lecanemab, another drug designed to slow the progression of Alzheimer’s. Although the study findings suggest Donanemab may outperform Lecanemab in terms of the extent to which it reduces Alzheimer’s brain pathology and the rate at which slows cognitive decline, the answer to the question of which is “better” (if Donanemab also receives full FDA approval) will ultimately depend on multiple factors. For example, how often a patient is able to receive infusions and what other health factors someone might have that influences the weighing of risks and benefits. That said, at minimum, the relative efficacy of these two drugs in early-stage Alzheimer’s underscores the potential of these approaches in successfully managing the disease. It also reassures researchers that they are still heading in the right direction.
Future Directions: Donanemab and the Landscape of Alzheimer’s Treatment
While the data on Donanemab are promising, we must bear in mind that it remains an experimental drug. Questions remain regarding side effects, costs and burden of screening, health system implications, and identification of the patient populations most likely to benefit from the treatment.
Nevertheless, the ability of Donanemab to potentially delay the progression of Alzheimer’s disease represents a significant advance. This development could have far-reaching implications, offering patients the opportunity for improved quality of life and extended cognitive function.
Eli Lilly, the manufacturer of Donanemab, has submitted the drug for FDA approval, with a decision expected by the end of the year. We eagerly anticipate the outcome of this process and the impact it could have on the treatment landscape of Alzheimer’s disease.
Donanemab and You
The emergence of Donanemab as a potential treatment for early-stage Alzheimer’s disease is significant for patients and their families, and its implications are multi-faceted.
1. Extended Quality of Life: Alzheimer’s is a progressive disease that gradually impairs cognitive function. By slowing the disease’s progression, Donanemab may enable patients to maintain their cognitive abilities for a more extended period. This prolongation could mean more time for patients to engage in activities they love, to connect with their loved ones, and to retain their independence.
2. Potential for Early Intervention: The findings again highlight the importance of early detection and treatment. The most significant benefits were observed in patients in the early stages of the disease. Thus, the development of Donanemab underscores the need for vigilant monitoring for early signs of Alzheimer’s and, if eligible, the initiation of treatment as early as possible.
3. More Hope Now and For the Future: These findings may offer some degree of comfort and relief specifically for family members. The potential of Donanemab to slow down the disease progression can provide families with the hope of having more quality time with their loved ones. For those concerned about an elevated risk of Alzheimer’s disease This time may not only allow for deeper connection and shared experiences, but also provide the opportunity to plan and prepare for the journey ahead.
4. Implications for Alzheimer’s Care: The advancement of Donanemab can potentially influence future care strategies for Alzheimer’s. Healthcare professionals and caregivers might be able to implement more personalized and effective care plans if patients can maintain a higher level of cognitive function for longer periods.
We, at Isaac Health, are committed to supporting you throughout your health journey. If you’d like further information about this breakthrough or to discuss potential Alzheimer’s treatment options, please do not hesitate to contact us.
As we closely monitor the progression of Donanemab through the approval process, we remain committed to providing the most current, comprehensive, and compassionate care. Each step forward in Alzheimer’s research brings us closer to improved patient care and outcomes, highlighting the importance of continued research and innovation in this critical field.
Sources
1. “Experimental Drug Donanemab Slows Progression of Early Alzheimer’s, Study Finds”. (2023, July 17). The Guardian. Link
2. “Donanemab in Early Symptomatic Alzheimer’s Disease.” (2023, July 17). JAMA. Link
3. “Experimental Alzheimer’s Drug Offers Hope of Slower Decline.” (2023, July 17). Nature. Link
4. “Understanding Genetics: APOE4.” (2019, April 12). The National Institutes of Health (NIH). Link
5. “FDA Grants Full Approval to Alzheimer’s Drug Lecanemab.” (2023, July 6). Reuters. Link